HTML
Research Article - (2021) Volume 11, Issue 9
Simultaneous Estimation of Sofosbuvir and Velpatasvir in Bulk and Dosage Form by UV Spectrophotometry
Raviraj Nagnath Jalkote**Correspondence: Raviraj Nagnath Jalkote, Department of Pharmacy, SPM’s College of Pharmacy, Maharashtra, India, Email:
Received: 12-Oct-2021 Published: 02-Nov-2021
Abstract
Simple, sensitive, specific and economic spectrophotometric method was developed and validated for simultaneous quantitation of sofosbuvir and velpatasvir in tablet dosage form. New method based on the simultaneous estimation of drugs in a binary mixture without previous separation was developed. In simultaneous equation method, sofosbuvir and velpatasvir were quantified using their absorptivity values of at selected wavelengths. The drug obeyed the Beer’s law and shows good correlation near to r2=0.999 for sofosbuvir 2r and for velpatasvir r2=0.998. Beer’s law was obeyed in concentration range of 5-25 μg/ml for sofosbuvir and 10-50 μg/ml for velpatasvir. The method has been validated for linearity, accuracy and precision. The recovery was 100.6% for sofosbuvir and 98.9% for velpatasvir. The developed method was found to be accurate, simple, precise, economical, and selective for simultaneous estimation of sofosbuvir and velpatasvir tablet dosage form.
Keywords
Sofosbuvir, Velpatasvir, Method validation, Estimation, Beer’s law.
Introduction
Sofosbuvir
The drug is used for the treatment of hepatitis C. It is only recommended for some combination of ribavirin, peginterferon-alfa, simeprevir, ledipasvir, or daclatasvir. Cure rates are 30%-97% depending on the type of hepatitis C virus involved. Safety during pregnancy is unclear; while, some of the medications used in combination may result in harm to the baby. It is taken by mouth.
Molecular formula: C22H29FN3O9P.
Molecular weight: 529.45 g/mol.
Pka: 9.3.
Mechanism of action: Sofosbuvir inhibits the hepatitis C NS5B protein. Sofosbuvir appears to have a high barrier to the development of resistance. It is metabolized to the active antiviral agent GS-461203 (2’-deoxy2’-α-fluoro-β-C-methyluridine-5’-triphosphate). GS-461203 serves as a defective substrate for the NS5B protein, which is the viral RNA polymerase, thus acts as an inhibitor of viral RNA synthesis. Although sofosbuvir has a 3’ hydroxyl group to act as a nucleophile for an incoming NTP, a similar nucleotide analog, 2’-deoxy-2’-α-fluoro-β- Cmethylcytidine, is proposed to act as a chain terminator because the 2’ methyl group of the nucleotide analog causes a steric clash with an incoming NTP (Figures 1 and 2) [1,2].
Velpatasvir
Velpatasvir is an NS5A inhibitor which is used together with Sofosbuvir in the treatment of hepatitis C infection of all six major genotypes.
Molecular formula: C49H54N8O8.
Molecular Weight: 883.02 g/mol.
Solubility: Soluble in water, methanol, and acetonitrile.
Pka: 3.74.
Indication: Used together with Sofosbuvir in the treatment of hepatitis C infection of all six major genotypes.
Mechanism of action: The substance blocks NS5A, a protein necessary for hepatitis C virus replication and assembly.
To our knowledge simple and economical analytical method for simultaneous determination of sofosbuvir and velpatasvir has not been reported so far. The present communication describes two simple, sensitive, accurate, rapid and economic methods for simultaneous estimation of sofosbuvir and velpatasvir in tablet formulation. The developed methods were validated and found to be accurate, precise and reproducible [3, 4].
Theory
Vierodt’s method of simultaneous equations: We can find out concentration of both the drug from combination mixture using the simultaneous equation method. In this method using the absorbance of both the drug and mixture at their wavelength and put this value in following equation and we can find out the concentration of drugs present in combination.
Cx=(A2 × Ay1)-(A1 × Ay2)………………………………..(1)
(Ay1 × Ax2)-(Ay2 × Ax1)
Cy=(A1 × Ax2)-(A2 × Ax1)……………………...................(2)
(Ax2 × Ay1)-(Ax1 × Ay2)
Where,
Cx=Concentration of drug X
Cy=Concentration of drug
A1=Absorbance of mixture at wavelength 1
A2=Absorbance of mixture at wavelength 2
Ax1=Absorptivity of drug A at wavelength 1
Ax2=Absorptivity of drug A at wavelength 2
Ay1=Absorptivity of drug B at wavelength 1
Ay2=Absorptivity of drug B at wavelength 2
The present study is to validate the UV spectrophotometric method for simultaneous estimation of sofosbuvir and velpatasvir in bulk and dosage form.
Materials and Methods
Selection of solvent
A number of trails were made to find out the ideal solvent system for dissolving the drugs. The solvents such as water, methanol and acetonitrile, n-hexane and ethanol were tried based on the solubility of the drugs. Sofosbuvir and velpatasvir are soluble in Methanol. Sofosbuvir and velpatasvir were dissolved in methanol, a clear solution was obtained. Better absorption maximum was found with methanol.
Instruments used: UV-visible spectrophotometer (shimadzu 1800 model). The UV-VIS spectrophotometer achieves a resolution of 1 nm with matched quartz cells of 1 cm path length.
Reagents and materials: API-sofosbuvir and velpatasvir pure drugs were purchased from Swapnroop Drugs and Pharmaceuticals, Aurangabad and Maharashtra in India. Tablets of 400 mg strength of sofosbuvir and 100 mg of velpatasvir were purchased from the local pharmacy in Solapur under commercially available brand name Velas of and methanol (99% AR) was used in this study.
Experimental work
Preparation of standard solution: Accurately weigh and transfer 10 mg of sofosbuvir and 10 mg of velpatasvir working standard into a 10 ml of clean dry volumetric flasks, add about 7 ml of diluent and sonicate to dissolve it completely and make up the volume up to the mark with the same solvent (Stock solution).
Further pipette out 0.3 ml of sofosbuvir and 0.15 ml velpatasvir from above stock solution into a 10 ml volumetric flask and dilute up to the mark with Methanol [5].
Procedure for assay of pharmaceutical formulations: Take average weight of one Tablet and crush in a mortar by using pestle and weight 10 mg equivalent weight of sofosbuvir and velpatasvir sample into a 10 ml clean dry volumetric flask and add about 7 ml of diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent.
Further pipette 0.3 ml of sofosbuvir and 0.15 ml velpatasvir above stock solution into a 10 ml volumetric flask and dilute up to the mark with methanol.
Preparation of drug solutions for linearity: Accurately weigh and transfer 10 mg of sofosbuvir and 10 mg of velpatasvir working standard into a 10 ml of clean dry volumetric flasks add about 7 ml of diluents and sonicate to dissolve it completely and make volume up to the mark with the same solvent (Stock solution).
Preparation of level-I (10 μg/ml of sofosbuvir and 5 μg/ml of velpatasvir): Pipette out 0.1 ml of sofosbuvir and 0.05 ml of velpatasvir from the stock solutions and transfer in a 10 ml of volumetric flask and dilute up to the mark with methanol (Diluent).
Preparation of level-II (20 μg/ml of sofosbuvir and 10 μg/ml of velpatasvir): Pipette out 0.2 ml of sofosbuvir and 0.1 ml of velpatasvir from the stock solutions and transfer in a 10 ml of volumetric flask and dilute up to the mark with diluent.
Preparation of level-III (30 μg/ml of sofosbuvir and 15 μg/ml of velpatasvir): Pipette out 0.3 ml of sofosbuvir and 0.15 ml of velpatasvir from the stock solutions and transfer in a 10 ml of volumetric flask and dilute up to the mark with diluent.
Preparation of level-IV (40 μg/ml of sofosbuvir and 20 μg/ml of velpatasvir): Pipette out 0.4 ml of sofosbuvir and 0.2 ml of velpatasvir from the stock solutions and transfer in a 10 ml of volumetric flask and dilute up to the mark with diluent.
Preparation of level-V (50 μg/ml of sofosbuvir and 25 μg/ml of velpatasvir): Pipette out 0.5 ml of sofosbuvir and 0.25 ml of velpatasvir from the stock solutions and transfer in a 10 ml of volumetric flask and dilute up to the mark with diluent.
Method development
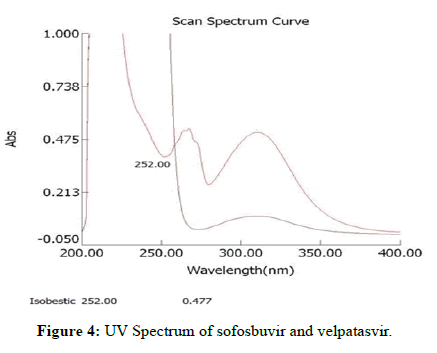
Sofosbuvir and velpatasvir show maximum solubility in methanol in comparison with other solvents. Hence methanol was chosen as a solvent (Table 1, Figures 3 and 4).
| S. No | Solvents | Solubility |
|---|---|---|
| 1 | Water | Slightly Insoluble |
| 2 | Methanol | Soluble |
| 3 | Acetonitrile | Sparingly soluble |
| 4 | Chloroform | Insoluble |
Method validation
The developed method was validated as per ICH guidelines for the following parameters;
Linearity: The linearity of an analytical procedure is its ability to obtain test results, which are directly proportional to the concentration of analyte in the sample. Linearity can be assessed by performing single measurements at several analyte concentrations. A linearity correlation coefficient above 0.9995 is acceptable for most methods, especially for major components in assay methods. The range of an analytical procedure is the interval between the upper and lower concentration of analyte in the sample.
Range: The Range of the analytical method was decided from the interval between upper and lower level of calibration curve by plotting curve [6].
Accuracy (Recovery studies): The accuracy of analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted true value. Accuracy studies were performed at three different levels (50%, 100% and 150%) by standard addition method and the samples were analysed in triplicate by the proposed method. Known amount of standard sofosbuvir and velpatasvir at 50%, 100% and 150 % of predetermined sample was added to a pre quantified tablet sample.
%Recovery=Observed value/True value × 100
Precision: The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. The precision of the method was determined in terms of repeatability and intra-day and inter-day precisions. Intraday precision was determined for both the drugs and each concentration for three times, on the same day. Interday precision was determined similarly, but the analysis being carried out daily, for two consecutive days. In the precision obtained five (5) replicates of 100% accuracy solution as per experimental conditions. Recorded the absorbance and calculated %RSD.
Robustness: The robustness of the developed method is its capacity to remain unaffected by small changes in altered conditions. To determine the robustness of the method, the wavelength of analysis was deliberate and the assay was evaluated. The effect of detection wavelength was studied at ± 5 nm [7].
Ruggedness: Ruggedness was determined by carrying out analysis by two different analysts and the respective absorbance was noted and the results were indicated as %RSD.
LOD and LOQ: The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be detected but not necessarily quantified as an exact value. The quantitation limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be quantitatively determined with suitable precision and accuracy. Limit of Detection and Limit of Quantitation were calculated using following formula;
LOD=3.3(SD)
LOQ=10 (SD)/S
Where,
SD=standard deviation of response (absorbance)
S=slope of the calibration
Results
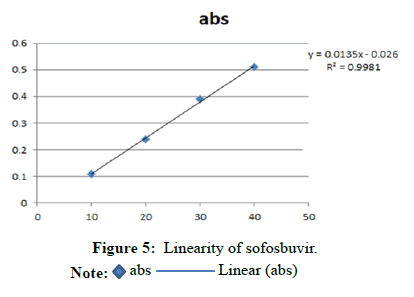
Linearity for sofosbuvir
(Table 2 and Figure 5)
| Concentration Level (%) | Concentration mg/ml | Absorbance |
|---|---|---|
| 33 | 5 | 0.168 |
| 66 | 10 | 0.343 |
| 100 | 15 | 0.48 |
| 133 | 20 | 0.642 |
| 166 | 25 | 0.807 |
Note: Regression equation=0.0312x+0.0185 r2=0.999
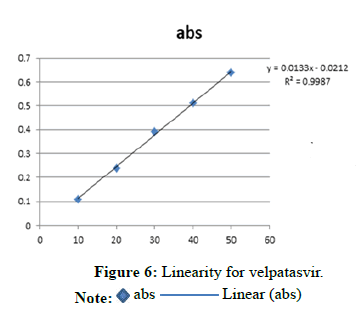
Linearity for velpatasvir (Tables 3-5 and Figure 6)
| Concentration Level (%) | Concentration mg/ml | Absorbance |
|---|---|---|
| 33 | 10 | 0.109 |
| 66 | 20 | 0.24 |
| 100 | 30 | 0.391 |
| 133 | 40 | 0.51 |
| 166 | 50 | 0.639 |
Note: Regression equation=0.0133x-0.0212 r2=0.9987
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Sofosbuvir | 0.474 |
| 2 | Sofosbuvir | 0.475 |
| 3 | Sofosbuvir | 0.475 |
| Mean | 0.475 | |
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Velpatasvir | 0.382 |
| 2 | Velpatasvir | 0.371 |
| 3 | Velpatasvir | 0.391 |
| Mean | 0.381 | |
%Assay=Sample abs/Standard abs × Weight of standard/Dilution of standard × Dilution of sample/Weight of sample × Purity/100 × Weight of tablet/Label claim × 100
%Assay=0.475/0.475 × 10/15 × 15/0.0569 × 99.6/100 × 2.8452/500 × 100
=99.8%
The % purity of sofosbuvir and velpatasvir in pharmaceutical dosage form was found to be 99.8%.
Accuracy (recovery study): (Tables 6 and 7)
| %Concentration(at specification level) | Absorbance | Amount added | Amount found(ppm) | % Recovery | Mean recovery |
|---|---|---|---|---|---|
| 50% | 0.247 | 15 | 14.8 | 98.6 | 100.60% |
| 100% | 0.483 | 30 | 29.6 | 98.6 | |
| 150% | 0.725 | 45 | 44.9 | 99.7 |
| %Concentration (at specification level) | Absorbance | Amount added (ppm) | Amount found (ppm) | % Recovery | Mean recovery |
|---|---|---|---|---|---|
| 50% | 0.166 | 7.5 | 7.5 | 100.2 | 98.90% |
| 100% | 0.378 | 15 | 15.1 | 100.1 | |
| 150% | 0.775 | 22.5 | 22.9 | 101.6 |
Precision: The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed condition.
Repeatability: Obtained five (5) replicates of 100% accuracy solution as per experimental conditions. Recorded the absorbance and calculated %RSD (Tables 8 and 9).
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Sofosbuvir | 0.474 |
| 2 | Sofosbuvir | 0.475 |
| 3 | Sofosbuvir | 0.475 |
| 4 | Sofosbuvir | 0.474 |
| 5 | Sofosbuvir | 0.474 |
| Mean | 0.4744 | |
| Std. Dev. | 0.000548 | |
| %RSD | 0.115456 | |
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Velpatasvir | 0.392 |
| 2 | Velpatasvir | 0.393 |
| 3 | Velpatasvir | 0.391 |
| 4 | Velpatasvir | 0.397 |
| 5 | Velpatasvir | 0.399 |
| Mean | 0.3944 | |
| Std. Dev. | 0.003435 | |
| %RSD | 0.870972 | |
Intermediate precision
(Table 10)
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Sofosbuvir | 0.493 |
| 2 | Sofosbuvir | 0.493 |
| 3 | Sofosbuvir | 0.494 |
| 4 | Sofosbuvir | 0.494 |
| 5 | Sofosbuvir | 0.493 |
| 6 | Sofosbuvir | 0.493 |
| Mean | 0.493333 | |
| Std. Dev. | 0.000548 | |
| %RSD | 0.111025 | |
Velpatasvir
(Tables 11 and 12)
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Velpatasvir | 0.377 |
| 2 | Velpatasvir | 0.378 |
| 3 | Velpatasvir | 0.378 |
| 4 | Velpatasvir | 0.372 |
| 5 | Velpatasvir | 0.376 |
| 6 | Velpatasvir | 0.374 |
| Mean | 0.375833 | |
| Std. Dev. | 0.002401 | |
| %RSD | 0.63895 | |
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Sofosbuvir | 0.454 |
| 2 | Sofosbuvir | 0.453 |
| 3 | Sofosbuvir | 0.454 |
| 4 | Sofosbuvir | 0.454 |
| 5 | Sofosbuvir | 0.454 |
| 6 | Sofosbuvir | 0.454 |
| Mean | 0.453833 | |
| Std. Dev. | 0.000447 | |
| %RSD | 0.098541 | |
Acceptance criteria: %RSD of six different sample solutions should not more than 2 (Table 13).
| S. no | Drug name | Absorbance |
|---|---|---|
| 1 | Velpatasvir | 0.371 |
| 2 | Velpatasvir | 0.374 |
| 3 | Velpatasvir | 0.379 |
| 4 | Velpatasvir | 0.373 |
| 5 | Velpatasvir | 0.371 |
| 6 | Velpatasvir | 0.373 |
| Mean | 0.3735 | |
| Std. Dev. | 0.00295 | |
| %RSD | 0.789713 | |
a. Limit of Detection (Table 14)
| LOD (μg/ml) | Con. |
|---|---|
| Sofosbuvir | 0.133 μg/ml |
| Velpatasvir | 0.852 μg/ml |
b. Limit of Quantification (Table 15)
| LOQ (µg/ml) | Con. |
|---|---|
| Sofosbuvir | 0.405 μg/ml |
| Velpatasvir | 2.582 μg/ml |
Robustness:
a. Sofosbuvir
• Variation in wavelength (Table 16)
| S. No | Drug name | Wavelength variation ± 1 | Absorbance |
|---|---|---|---|
| 1 | Sofosbuvir | 251(less) | 0.469 |
| 2 | Sofosbuvir | 252(actual) | 0.478 |
| 3 | Sofosbuvir | 253(more) | 0.47 |
%RSD= 1.04436
b. Velpatasvir
• Variation in wavelength (Table 17)
| S. No | Drug name | Wavelength variation±1 | Absorbance |
|---|---|---|---|
| 1 | Velpatasvir | 251 (less) | 0.375 |
| 2 | Velpatasvir | 252 (actual) | 0.388 |
| 3 | Velpatasvir | 253 (more) | 0.391 |
%RSD=1.210979
Ruggedness: ( Table 18)
| Analyst | Absorbance | |
|---|---|---|
| Sofosbuvir | Velpatasvir | |
| Analyst 1 | 0.458 | 0.376 |
| Analyst 2 | 0.465 | 0.38 |
| Analyst 3 | 0.46 | 0.374 |
| %RSD | 0.782115 | 0.811075 |
Discussion
Preliminary analysis of sofosbuvir and velpatasvir
Preliminary analysis of sofosbuvir and velpatasvir such as description, solubility was performed.
Assay of tablet formulation
Amount of drug present in tablet formulation was calculated using simultaneous equation for sofosbuvir and velpatasvir respectively, and 0.0312 x+0.0185 and 0.0133 x-0.0212 for sofosbuvir and velpatasvir respectively. Amount of sofosbuvir and velpatasvir were found to be 99% of label claim. This method can be employed for routine analysis of both the drugs [8, 9].
Summary
Summary of UV spectrophotometric method for sofosbuvir and velpatasvir (Table 19).
| S. no. | Parameters | Values | |
|---|---|---|---|
| Sofosbuvir | Velpatasvir | ||
| 1 | Beer’s Law limit (μg/ml) | May-25 | Oct-50 |
| 2 | Isobestic point/ wavelength | 252 nm | 252 nm |
| 3 | Standard regression equation | 0.0312x+0.0185 | 0.0133x-0.0212 |
| 4 | Correlation coefficient (r2) | 0.999 | 0.998 |
| 5 | Accuracy (Isobestic point method) | 98.6-99.7 | 100.2-101.6 |
| 6 | Precision (% RSD) repeatability | 0.000548 | 0.870972 |
| 7 | LOD (μg/ml) | 0.133 μg/ml | 0.852 μg/ml |
| 8 | LOQ (μg/ml) | 0.405 g/ml | 2.582 μg/ml |
| 9 | Robustness (%RSD) | 1.044365 | 1.210979 |
| 10 | Ruggedness (%RSD) | 0.782115 | 0.811075 |
| 11 | Assay (%) | 99.80% | 99.80% |
Conclusion
The UV-spectrophotometric method was developed and it is found to be simple, accurate, precise, highly sensitive, reproducible and inexpensive. The proposed method was found suitable for determination of sofosbuvir and velpatasvir in API and its dosage form without any interference from the excipients. This method can be effectively applied for the routine analysis of sofosbuvir and velpatasvir in API. Its advantages are the low cost of reagents, speed and simplicity of sample treatment, satisfactory precision and accuracy.
Acknowledgement
The authors are very thankful to the Principal of SPM’s College of Pharmacy, Malewadi-akluj Sholapur, Maharashtra, India and cooperative staff for providing the required facilities and guidance to carry out this research work.
References
- Nekkala K, Shanmukha Kumar JV, Ramachandran D. Asian. J. Pharm. Clin. Res. 2018; 11(2): 164-168.
- National center for biotechnology information. Pub.Chem. 2020.
- National center for biotechnology information. Pub.Chem. 2019.
- Velpatasvir. Drug. Bank. 2019.
- Validation of analytical procedure: text and methodology. ICH. 2005.
- Chakravarthy A, Bbv S, Kumar P. Asian. J. Pharm. Clin. Res. 2016; 9: 61-66.
- Nemade MR. World. J. Pharm. Pharma. Sci. 2017; 749-757.
- Zaman B, Siddique F, Hassan W. Chromatographia. 2016; 79: 1605-1613.
- Sofosbuvir.Wikipedia. 2019.
Manuscript Submission
Submit your manuscript at Online Submission System
Google scholar citation report
Citations : 1101
International Journal of Pharmacy received 1101 citations as per google scholar report
International Journal of Pharmacy peer review process verified at publons
Indexed In
- CAS Source Index (CASSI)
- HINARI
- Index Copernicus
- Google Scholar
- The Global Impact Factor (GIF)
- Polish Scholarly Bibliography (PBN)
- Cosmos IF
- Open Academic Journals Index (OAJI)
- Directory of Research Journal Indexing (DRJI)
- EBSCO A-Z
- OCLC- WorldCat
- MIAR
- International committee of medical journals editors (ICMJE)
- Scientific Indexing Services (SIS)
- Scientific Journal Impact Factor (SJIF)
- Euro Pub
- Eurasian Scientific Journal Index
- Root indexing
- International Institute of Organized Research
- InfoBase Index
- International Innovative Journal Impact Factor
- J-Gate
 (abs)
(abs)